Engineering synthetic folded nanoarchitectures
Professor Ivan Huc, European Institute of Chemistry & Biology University of Bordeaux, France
Info about event
Time
Location
iNANO Auditorium (1593-012), Gustav Wieds Vej 14, 8000 Aarhus C

Professor Ivan Huc, European Institute of Chemistry & Biology
University of Bordeaux, France
Engineering synthetic folded nanoarchitectures
Abstract: Aromatic amide oligomers constitute a new, distinct, and promising class of synthetic foldamers – oligomers that adopt stable folded conformations. Single helical structures are predictable, show unprecedented conformational stability, and constitute convenient building blocks to elaborate synthetic, very large (protein-sized) folded architectures (Fig. 1). They possess a high propensity to assemble into double, triple and quadruple helices, or to fold into sheet-like structures. Cavities can be designed within such synthetic molecules that enable them to act as artificial receptors and molecular motors. Water soluble analogues of these foldamers show promise in nucleic acid and protein recognition. This lecture will give an overview of the design principles of these functional molecular architectures and of their associated dynamics, including folding-unfolding equilibria, guest binding and release as well as translational and rotational motions.
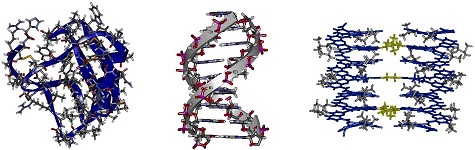
Fig. 1. Crystal structures shown at the same scale of an 8 kDa protein (left), B-DNA (center) and an aromatic foldamer helix bundle (right).
References:
- G. Guichard, I. Huc. Synthetic foldamers. Chem. Commun. 2011, 47, 5933
- Q. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grélard, H. Jiang, I. Huc. Helix-rod host-guest complexes with shuttling rates much faster than disassembly. Science 2011, 331, 1172
- Q. Gan, Y. Ferrand, N. Chandramouli, B. Kauffmann, C. Aube, D. Dubreuil, I. Huc. Identification of a foldaxane kinetic byproduct during guest- induced single to double helix conversion. J. Am. Chem. Soc. 2012, 134, 15656
- L. Sebaoun, V. Maurizot, T. Granier, B. Kauffmann, I. Huc. Aromatic Oligoamide β-Sheet Foldamers. J. Am. Chem. Soc. 2014, 136, 2168
- T. Qi, T. Deschrijver, I. Huc. Large-scale and chromatography-free synthesis of an octameric quinoline-based aromatic amide helical foldamer. Nature Protocols 2013, 8, 693
- N. Chandramouli, Y. Ferrand, G. Lautrette, B. Kauffmann, C. D. Mackereth, M. Laguerre, D. Dubreuil, I. Huc. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nature Chemistry, 2015, 7, 334