Cryo-microscopy reveals nano-sized copy machine implicated in origin of life
RNA is thought to have sparked the origin of life by self-copying. Researchers from Aarhus University, Denmark, and MRC LMB Cambridge, England, have revealed the atomic structure of an "RNA copy machine" through cryo-EM. This breakthrough sheds light on a primordial RNA world and fuels advancements in RNA nanotechnology and medicine.
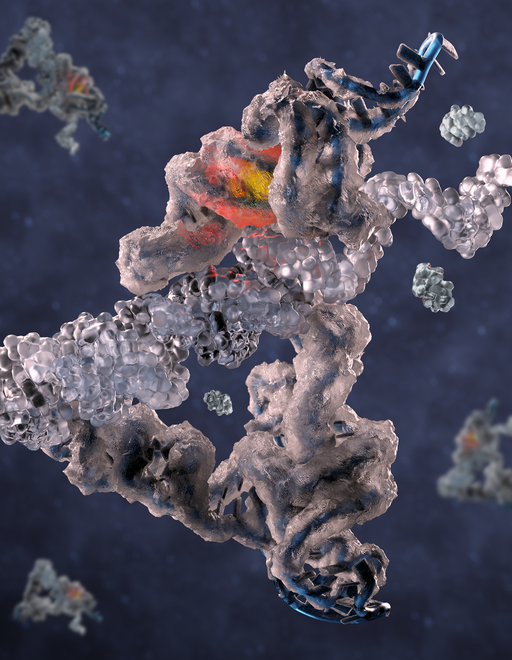
How the intricate molecular machinery of life arose from simple beginnings has been a long-standing question. Several lines of evidence point towards a primordial "RNA world", where an "RNA copy machine" (a so-called replicase) started making copies of itself and other RNA molecules to kick-start evolution and life itself. However, the ancient replicase appears to have been lost in time and its role in modern biology has been taken over by more efficient protein machines. To support the RNA world hypothesis, researchers have been seeking to re-create an equivalent of the RNA replicase in the laboratory. While such molecular “Doppelgangers” of the ancient replicase have been discovered, both their detailed molecular structure and mode of action has remained elusive due to the difficulty of determining the structure of dynamic RNA molecules.
Structure of an ice-loving RNA replicase
In a research paper published in PNAS, a team of researchers now report the first atomic structure of an RNA replicase using cryogenic electron microscopy (cryo-EM). The RNA replicase being studied was developed by the Holliger lab (MRC LMB Cambridge, UK) to be efficient at copying long templates using nucleotide triplets in the eutectic ice phase (similar to slush-ice). Returning from postdoctoral studies in the Holliger lab, Emil L. Kristoffersen, currently assistant professor at Aarhus University, facilitated a collaboration with the Andersen lab (Aarhus University, Denmark) to determine the structure of the RNA replicase by cryo-EM. Interestingly, the structure shows striking similarities to protein-based polymerases with domains for template binding, polymerization, and substrate discrimination arranged in a molecular shape resembling an open hand.
"It was surprising to find that a ribozyme that we evolved artificially in the test tube would display features of naturally occurring protein polymerases. This indicates that evolution can discover convergent molecular solutions no matter if the material is RNA or protein", explains Philipp Holliger, program leader at MRC LMB Cambridge, UK.
Model for RNA synthesis in an RNA world
To better understand how the RNA replicase works, the researchers did a comprehensive mutational study to highlight the crucial elements of the RNA structure. This analysis confirmed features of the catalytic site but also revealed the importance of two so-called kissing-loop interactions, which bind the scaffolding and the catalytic subunits together, as well as the importance of a specific RNA domain for fidelity, that is the accuracy with which the replicase copies RNA strands. While the researchers could not determine the structure of the replicase “in-action” while actively copying RNA, it was possible to build a model for RNA-based RNA copying that is consistent with all experimental data.
"Cryo-EM is a powerful method for studying the structure and dynamical features of RNA molecules. By combing cryo-EM data with experiments, we were able to build a model of the inner workings of this complex RNA machine", tells Ewan McRae, who did the cryo-EM work as a postdoc in the Andersen lab at Aarhus University but has now started his own research group at Houston Methodist Research Institute, Texas, USA.
Inspiration for RNA nanotechnology and medicine
The study provides an exciting first glimpse of an RNA replicase thought to reside at the very root of the tree of life. The currently developed RNA-based replicases are however very inefficient (as compared to protein-based polymerases) and cannot yet sustain their own replication and evolution. The structural insight provided by the reported study may help in designing more efficient replication mechanisms and thus get us closer to developing RNA world scenarios in the test tube.
"The properties of RNA replicases may be further improved by using chemical modifications that could exist in an RNA world. In addition, research into the origin of life leads to the discovery of several novel RNA building blocks that may be used in the emerging field of RNA nanotechnology and medicine", explains Ebbe Sloth Andersen, associate professor at Aarhus University, Denmark.
About the research
Study type:
Experimental Molecular Biology
External funding:
The research was funded by Independent Research Fund Denmark (9040-00425B), Novo Nordisk Foundation (NNF21OC0070452), Canadian Natural Sciences and Engineering Research Council (532417), Carlsberg Foundation (CF20-0635, CF17-0809), Lundbeck Foundation (R250-2017-1502), Medical Research Council, as part of United Kingdom Research and Innovation (also known as UK Research and Innovation (UKRI)) (MC_U105178804), Volkswagen Foundation (96 755), Herchel Smith studentship (2017), and Marie Curie fellowship (H2020-MSCA-IF-2018-845303).
Conflicts of interest:
The researchers declare that there are no conflicts of interest in connection with this research.
Link to the scientific article:
Cryo-EM structure and functional landscape of an RNA polymerase ribozyme
Ewan K. S. McRae, Christopher J. K. Wan, Emil L. Kristoffersen, Kalinka Hansen, Edoardo Gianni, Isaac Gallego, Joseph F. Curran, James Attwater, Philipp Holliger, and Ebbe S. Andersen
Proceedings of the National Academy of Sciences (PNAS) Vol. 121, No. 2 (January 9, 2024)
https://doi.org/10.1073/pnas.2313332121
Contact information:
Ebbe Sloth Andersen
Email: esa@inano.au.dk
Phone: +45 41178619
Philipp Holliger
Email: ph1@mrc-lmb.cam.ac.uk
Phone: +44 1223 267092