Joint Distinguished iNANO lectures by Prof. Dr. Mireille Claessens, University of Twente & Prof. Vito Foderà, University of Copenhagen
Info about event
Time
Location
iNANO AUD (1593-012)
Organizer
Alpha-synuclein and the random coil conundrum: exploring the role of an IDP in membrane remodelling and disease
The talk focuses on the intrinsically disordered protein alpha-synuclein, its role in membrane remodelling, and involvement in neurodegenerative diseases, highlighting the role of chaperone proteins in preventing harmful aggregation.
In proteins, fundamental to life's processes, 3D structure and function are strongly coupled. Intrinsically disordered proteins (IDPs) defy this structure-function paradigm. Our studies show that in solution the IDP alpha-synuclein (aS) behaves very close to a random coil polymer, it is truly an IDP. The random coil nature of aS is probably exploited by cells for membrane remodelling by exerting lateral pressure. Disorderness however also gives rise to problems. Many IDPs, including aS, are involved in the development of neurodegenerative diseases where the IDPs aggregate into amyloid fibrils. Nature prevents this from happening using chaperone proteins by acting at a very early stage of protein condensation. The formation of chaperone/IDP co-condensates modulates the energy landscape and thus deflects harmful IDP aggregation.
This study delves into molecular nanoscience, examining the functional roles and prevention mechanisms of intrinsically disordered proteins in disease-related nanoscale aggregation.
Ion-induced Phase Separation and Amyloid Morphologies
Professor Vito Foderà, Department of Pharmacy, University of Copenhagen
The talk will focus on the capability of a model globular protein, insulin, to undergo phase separation in presence of salt ions and how salt ions can determine different amyloid morphologies during in vitro aggregation experiments.
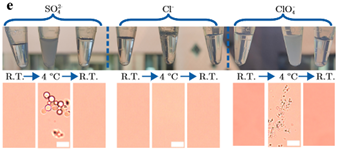
Amyloid reactions are regulated by a delicate balance between the hydrophobic attraction and the electrostatic barrier to aggregation. Prior to the onset of aggregation, the presence of ions in protein solutions can give rise to complex phase diagrams resulting in, for example, coacervation, liquid-liquid phase separation (LLPS), and re-entrant condensation. Using microscopy, small-angle X-ray scattering and atomistic molecular dynamics simulations, we report the mechanisms by which H-bonds and electrostatic interactions in ion-protein systems determine phase separation at low temperatures [1]. The effect is salt-specific, and, under aggregation conditions, the anion-protein interactions translate into the activation of a coalescence process, leading to amyloid-like microparticles alternative to amyloid fibrils [1, 2].
We bring about the concept of amyloid morphology encoded in LLPS propensity. Modifying the inter-protein interactions at the nanoscale during LLPS may define strategies to control the amyloid morphology during the solid transition.
References
[1] Lenton S. et al. 2024, bioRxiv https://doi.org/10.1101/2024.01.10.574993
[2] Chaaban H et al. 2022 J. Phys. Chem. Lett. 13, 16, 3586–3593