Chemicals
Working with chemicals
Avoid contact with chemicals in all situations where chemicals are handled: Weighing out, pouring, routine lab work, transporting, cleaning up and disposal of waste.
- Avoid any contact of chemicals and solvents with the skin or eyes.
- Wear lab coat, gloves and safety goggles.
- Avoid breathing in chemicals and vapours. Always work in the fume cupboard.
- Weighing out of chemicals must always be carried out in a fume cupboard or point suction.
- Wipe up any spills immediately.
Storing chemicals
- Chemicals must be stored in closed, clearly labelled containers: Name, formula, melting point/boiling point.
- Possible hazardous characteristics: Explosive, inflammable, self-igniting, water or air sensitive, caustic, poisonous, allergenic or carcinogenic.
- Solutions in ether and other volatile solvents can only be stored in the refrigerator when explosion-safe. Beakers must not be used. Use only closed flasks and bottles.
- Only small amounts of inflammable chemicals/solvents can be stored in the laboratory.
Transporting chemicals
- Chemicals transported out of the laboratory must be in closed containers. Glass containers must be carried in a carrying basket or on a trolley.
- Specially volatile, fuming, caustic, inflammable and explosive chemicals must not be transported in a manned elevator. This applies for example to volatile solvents, liquid nitrogen, dry ice and fuming acids.
Chemical spillage
- Spilled chemicals must be wiped up immediately. Liquids are absorbed by porous material (cat litter, sand, vermiculite, etc.) after neutralising and /or diluting with water. Spilled powder can be dusty to wipe up, therefore use suitable personal protection must be used. For disposal, see under the section Waste Disposal.
- Contaminated clothing should be changed as quickly as possible. Shoes, watch straps, etc. that have absorbed liquids should be removed immediately.
Peroxides and other unstable substances
For substances or containers with possible explosive qualities, age, storage temperature, light and air can be crucial for stability, so it is of utmost importance that these substances are not bought and stored in large quantities.
The Danish Emergency Management Agency (”Beredskabsstyrelsen”) has complete information on peroxides and formic acid.
If in doubt about high peroxide contents then be very careful about carrying and opening the container. A simple method of checking whether there are peroxides in e.g. ether is to mix a couple of mls with a potassium-iodide solution, add a couple of drops of diluted HCl and shake. The brown colour of iodine is a sure sign of peroxide.
The peroxide content can be checked with Peroxide Strips (Merck 1.10081.0001, level 1- 100 mg/L H2O2). Most peroxide-forming chemicals carry a stabiliser when delivered and chemical companies usually guarantee the shelf-life in unopened containers for three to five years from the production date. For chemicals not containing added stabilisers, there is a shorter shelf life.
Some peroxide-forming substances can reach explosive peroxide levels without a concentration of the solvent, and the general rule for substitution means that there should be a special reason for using diisopropylether.
According to the Danish “ADR” rules for transporting dangerous material, many of the ethers used routinely are classified as class 3 inflammable liquids. These rules (article 2.2.3.2.1) state that Class 3 liquids that easily form peroxides can only be transported by road when the peroxide content is no more than 0.3%, i.e. 3000mg/L. Such a high peroxide content will seldom be found in a laboratory, and unused peroxide-forming substances will normally be disposed of via the waste disposal system.
If in doubt – or it is known – that a substance has a high peroxide content (limit 100ppm), contact an Occupational Health and Safety representative/supervisor for further information, e.g. it could mean destroying the peroxides with an acid solution of ferrous- sulfate.
Containers with unstable chemicals should be labelled with date of purchase, date of opening, stability control, location, etc.
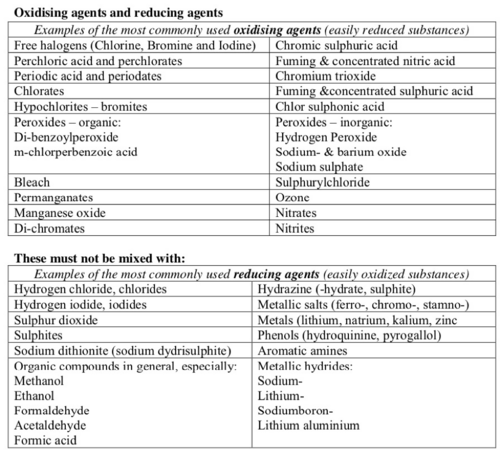
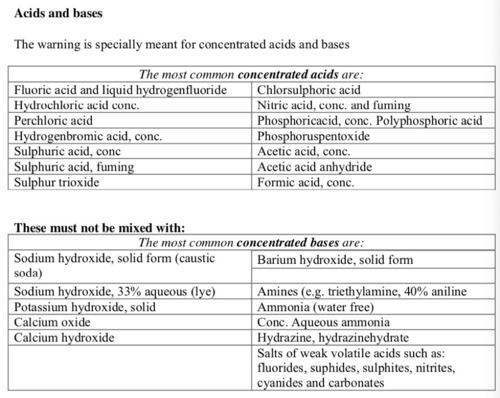
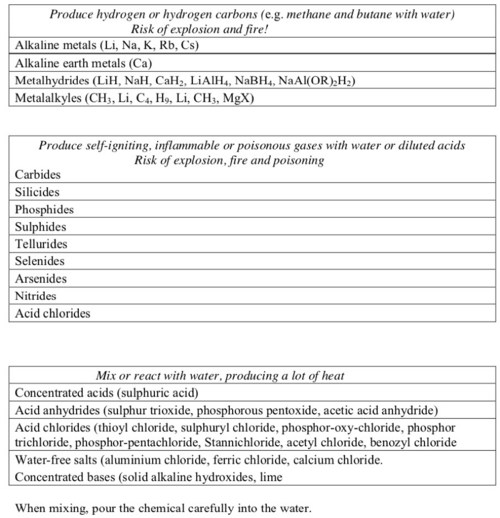
List of incompatible substances
Unless the mixing process is under control do not mix:
- oxidising agents and reducing agents
- acids and bases
- water-reacting substances and water.