Advancing the technology on electrochemical water splitting for sustainable energy
AU researchers reveal new atomic-scale insight into the active interface between Cobalt oxides and gold in the effort to optimize the technology of splitting water, which has the potential of providing an almost unlimited source of renewable resources.
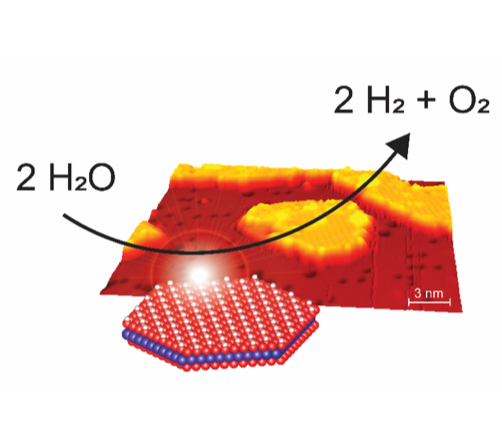
The successful implementation of sustainable energy technologies such as wind turbines and solar cells in recent decades means that it will become increasingly attractive to electrify many energy demands, which are today powered by fossil molecules. Using electricity to split water into oxygen and hydrogen in electrolysis is one of such attractive technologies, but a challenge is the large amounts of expensive Pt, Ir and Ru metals currently needed to catalyze the reaction. Oxides of cheap and abundant 3d metals (Co, Fe, Ni) are proven catalysts for the reaction, but more insight is needed to develop better catalysts from this group of inexpensive metals.
In the new publication in Angewandte Chemie, the group of Jeppe V. Lauritsen collaborated with the Max-Planck Institute for Solid State Research in Stuttgart to reveal new atomic-scale insight into the active interface between CoOx and gold. The results were obtained by combining atom-resolved imaging with a scanning tunneling microscope and an electrochemical cell which measures the efficiency of the catalyst. In this way, it was possible to link important nanoscale parameters to the catalytic function, thereby highlighting how gold nanoparticles can act as a stabilizer of the most active CoOx nanostructure. The work also highlights that a key parameter for catalytic may be the availability of edge sites on CoOx nanostructures.
The main author of the paper Jakob Fester from the Interdisciplinary Nanoscience Center defended his PhD thesis on the topic in June 2018.
This work was financially supported by Aarhus University Center for Integrated Materials Research (iMAT), Lundbeck Foundation and Villum Foundation.
The research has been carried out by researchers from Interdisciplinary Nanoscience Centre (iNANO) and Department of Physics and Astronomy at Aarhus University in collaboration with Brno University of Technology, Universidad Nacional de LaPlata, Max Planck Institute for Solid State Research, Max Planck Institute for Solid State Research and cole Polytechnique Federale de Lausanne. Associate Professor Jeppe Vang Lauritsen has been in charge of the research team behind the study.
For further information, please contact
Associate Professor Jeppe Vang Lauritsen
Interdisciplinary Nanoscience Center and Department of Physics and Astronomy
jvang@inano.au.dk - +45 23382369