(B)reaking (B)onds - diboron reagents reducing water to hydrogen (H2)
Skrydstrup Group publishes in Angewandte Chemie International Edition about the efficient reduction of water to dihydrogen by using diboron compounds
Recent years have witnessed a widespread increase for the exploitation of certain diboron agents. Here the Skrydstrup group has experienced that they can be used for breaking up water molecules resulting in dihydrogen (H2).
As H2 is flammable and dangerous to handle in larger amounts this finding may prove valuable in generating H2 in small amounts. This can further be used for the important reaction of reducing double bonds between carbon atoms in organic synthesis.
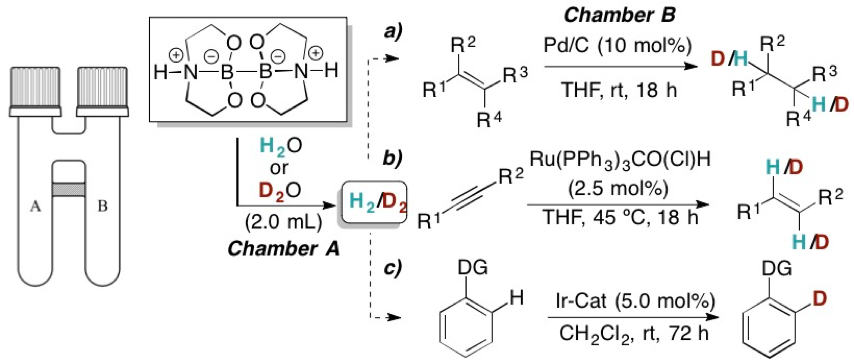
Mathias Flinker Dr. Hongfei Yin, René W. Juhl, Dr. Espen Z. Eikeland, Senior Researcher Jacob Overgaard, Dr. Dennis U. Nielsen and Prof. Troels Skrydstrup report on the preparation of a series of crystalline sp3-sp3 diboron(4) compounds, which are capable of reducing water to dihydrogen without the need for additives or transition metal complexes for activation. The role of the pendant nitrogen atoms and the sp3-hybridization of both boron atoms were shown to be crucial for obtaining the desired reactivity. The applicability of these compounds in synthesis as a simple and stoichiometric H2 or D2 source was demonstrated in a range of hydrogenations of olefins, semi-hydrogenations of alkynes and hydrogen-deuterium exchange reactions in combination with our two-chamber technology. Add the diboron compound to water or D2O and within a few seconds H2 or D2 is formed. In addition, the intermediacy of a borohydride species could be exploited for aldehyde/ketone reductions with the hydride source originating from water.
For direct access to the paper:
http://onlinelibrary.wiley.com/doi/10.1002/anie.201709685/full
This chemistry is funded by the Danish National Research Foundation and involves the two DNRF centers, CADIAC and CMC.