How small can you make nucleic acid nanostructures and why does it matter?
Researchers from the interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry at Aarhus University have significantly advanced the field of nucleic nanostructures, potentially transforming their application in biomedicine.
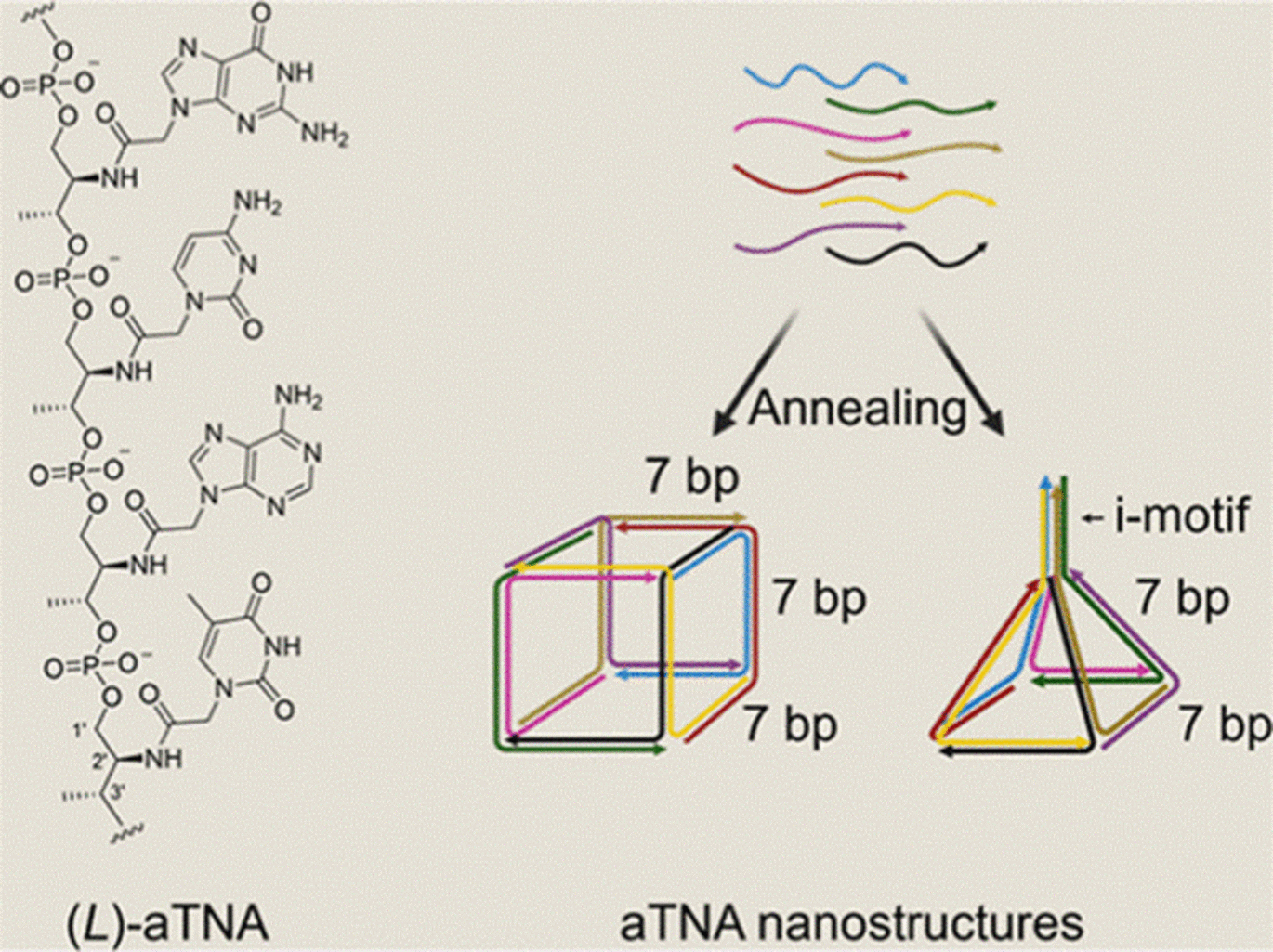
The research — recently published in the Journal of the American Chemical Society — was led by former PhD student Mads Kock Skaanning under the supervision of Professor Kurt V. Gothelf. The study introduces a pioneering approach using the amino acid-derived acyclic (L)-threoninol nucleic acid (aTNA), which forms unusually stable helices, enabling the creation of smaller nanostructures. Composed of multiple aTNA strands, these structures have the potential to integrate multiple drugs and targeting ligands, paving the way for multifunctional therapeutics.
The primary advantage of reducing the size of these structures to below 5 nm is their enhanced ability to penetrate tissues more effectively. This property is critical for targeted drug delivery systems, where the ability to reach specific types of cells or intracellular environments can significantly impact the efficacy of treatments, particularly in densely packed tissues such as tumors or the brain.
The enhanced penetration capabilities are complemented by aTNA's robustness. Traditional DNA and RNA nanostructures are often limited by their susceptibility to enzyme degradation and instability under varying ionic strengths. aTNA's resilience under physiological conditions ensures that these smaller structures do not sacrifice durability for size. Instead, they maintain their integrity within the body for longer, allowing for more precise and sustained therapeutic actions.
Mads Kock Skaanning expressed enthusiasm about the project's potential impact: "Our work with aTNA not only challenges existing limitations in nanostructure design but also opens up exciting possibilities for targeted drug delivery and precision medicine.".
Professor Kurt Gothelf, emphasized the broader implications of their findings: "This advancement not only enhances the practicality of nanostructures in medical applications but also sets a new standard for the construction of smaller, yet robust, functional nanoscale devices.".
The development of aTNA and its application in stable, functional nanostructures represents a significant leap forward, offering new tools for the development of targeted therapies and diagnostic methods. The team at Aarhus University continues to explore the possibilities opened up by this technology, aiming to further integrate these nanostructures into practical medical applications.
About the research
Study type:
Nucleic acid nanotechnology, Experimental chemistry
External funding:
The work is funded by the Novo Nordisk Foundation (CEMBID) (grant number NNF17OC0028070).
Conflicts of interest:
The authors declare no competing financial interest.
Link to the scientific article:
Self-Assembly of Ultrasmall 3D Architectures of (l)-Acyclic Threoninol Nucleic Acids with High Thermal and Serum Stability
Mads K. Skaanning, Jonas Bønnelykke, Minke A. D. Nijenhuis, Anirban Samanta, Jakob Melgaard Smidt & Kurt V. Gothelf
J. Am. Chem. Soc. 2024, 146, (29), 20141-20146, Self-Assembly of Ultrasmall 3D Architectures of (l)-Acyclic Threoninol Nucleic Acids with High Thermal and Serum Stability | Journal of the American Chemical Society (acs.org)
Contact information:
Professor Kurt Vesterager Gothelf
Aarhus University
Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry
Email: kvg@chem.au.dk