Nickel-Catalyzed Innovation in Drug Labeling
In a major advancement for pharmaceutical science, researchers from iNANO, in collaboration with AstraZeneca and The Arctic University of Norway (UiT), have developed a novel method for synthesizing isotopically labeled alkyl aryl ketones.
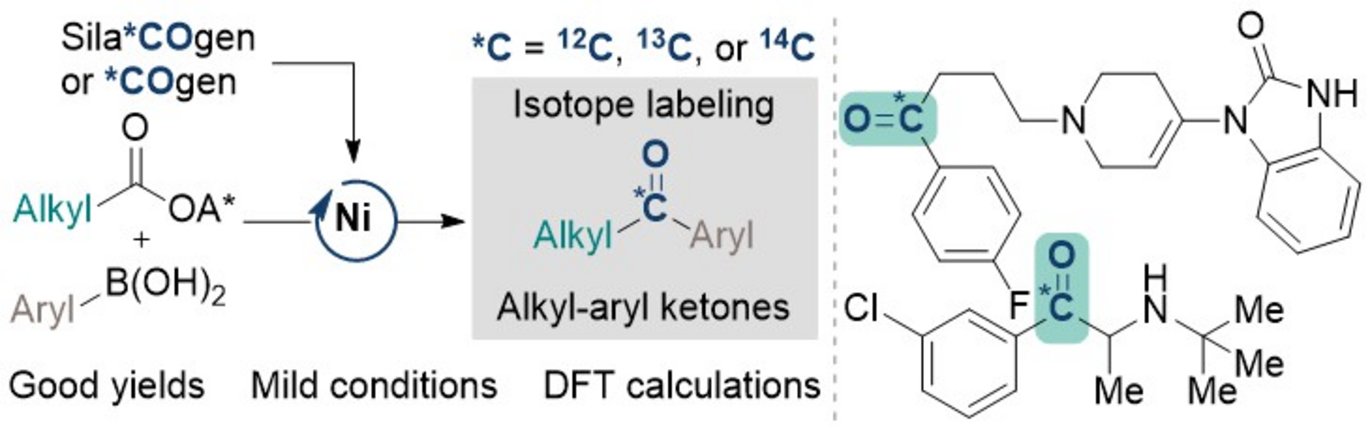
The study published in Angewandte Chemie reveals an efficient way to incorporate stable (13C) and radioactive carbon isotopes (14C) directly into pharmaceutical compounds. The methodology employs nickel catalysts and aryl boronic acids, combined with redox-activated alkyl carboxylic esters under mild conditions, to create isotopically labeled ketones, a key structure found in many drugs. This approach could aid the production of labeled pharmaceuticals, particularly in late-stage isotope labeling, which is critical for drug development.
The use of COgen and SilaCOgen allow easy switching between stable and radioactive isotopes and marks a significant advantage for this reaction. Radiolabeled compounds, which are crucial for tracing a drug's behavior, are notoriously difficult and expensive to produce. This method offers a simpler solution for incorporating carbon isotopes, reducing reliance on complex radiolabel precursors.
The researchers conducted detailed Density Functional Theory (DFT) calculations and experimental validation, confirming that the process involves carbon-centered radical formation and a CO insertion step—crucial for producing the ketone products.
These advancements allow pharmaceutical companies to generate essential metabolism data in drug development, providing insights into how drugs behave in the body and their potential environmental impact. The technique also supports labeling for clinical pharmacokinetic and pharmacodynamic studies.
The method has already been successfully applied to pharmaceuticals such as bupropion and droperidol, illustrating its versatility across different drug types.
About the research
Study type:
Experimental Chemistry
External funding:
KHH and SG thank the Research Council of Norway (No. 300769) and Sigma2 (No. nn9330k and nn4654k). KSM, KHH and SG thank the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 859910. VE thanks the Danish National Research Foundation (No. DNRF118)
Conflicts of interest:
Troels Skrydstrup is a co-owner of SyTracks a/s, which commercializes the COware® technology.
Link to the scientific article:
https://doi.org/10.1002/anie.202412247
Kim S. Mühlfenzl, Vitus J. Enemærke, Sahil Gahlawat, Peter I. Golbækdal, Nikoline Munksgaard-Ottosen, Karoline T. Neumann, Kathrin H. Hopmann, Per-Ola Norrby, Charles S. Elmore and Troels Skrydstrup.
Contact information:
Professor Troels Skrydstrup
Aarhus University
Interdisciplinary Nanoscience Center (iNANO)
Department of Chemistry
Email: ts@chem.au.dk